2024 ‘DDF In Action’ Day
TransCelerate had an in-person full day event:
DDF IN ACTION
Transforming Clinical Trials with Standards and Digitalization:
Continuing the Journey, Charting the Future
Overview
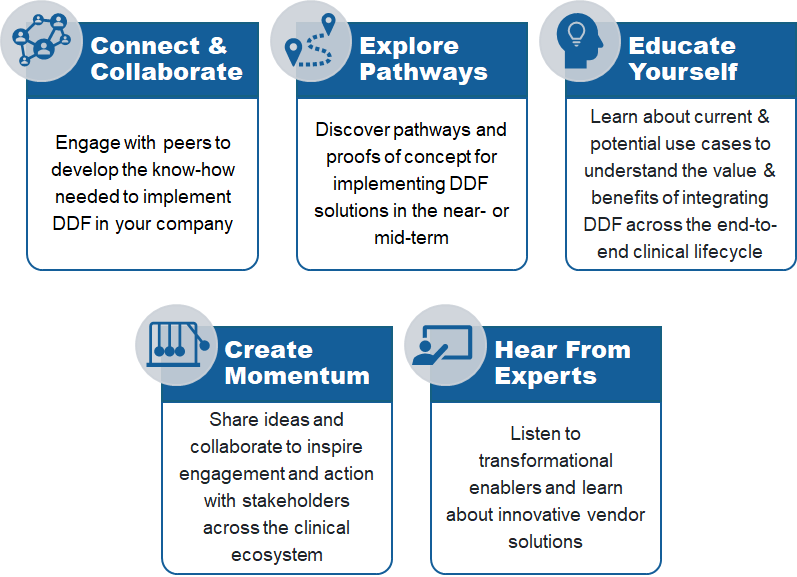
This interactive in-person experience brought together sponsor companies, clinical solution providers, and key industry stakeholders to exchange knowledge and collaborate on implementing the Unified Study Definition Model (USDM) and Study Definitions Repository (SDR) DDF solutions.
Date & Locations
Date: Thursday, October 10th, 2024
Locations: Europe & US
- Copenhagen, Denmark: Hosted by Novo Nordisk
- New Jersey, US: Hosted by Johnson & Johnson
Objectives